What Are the 3 Subatomic Particles Describe Each
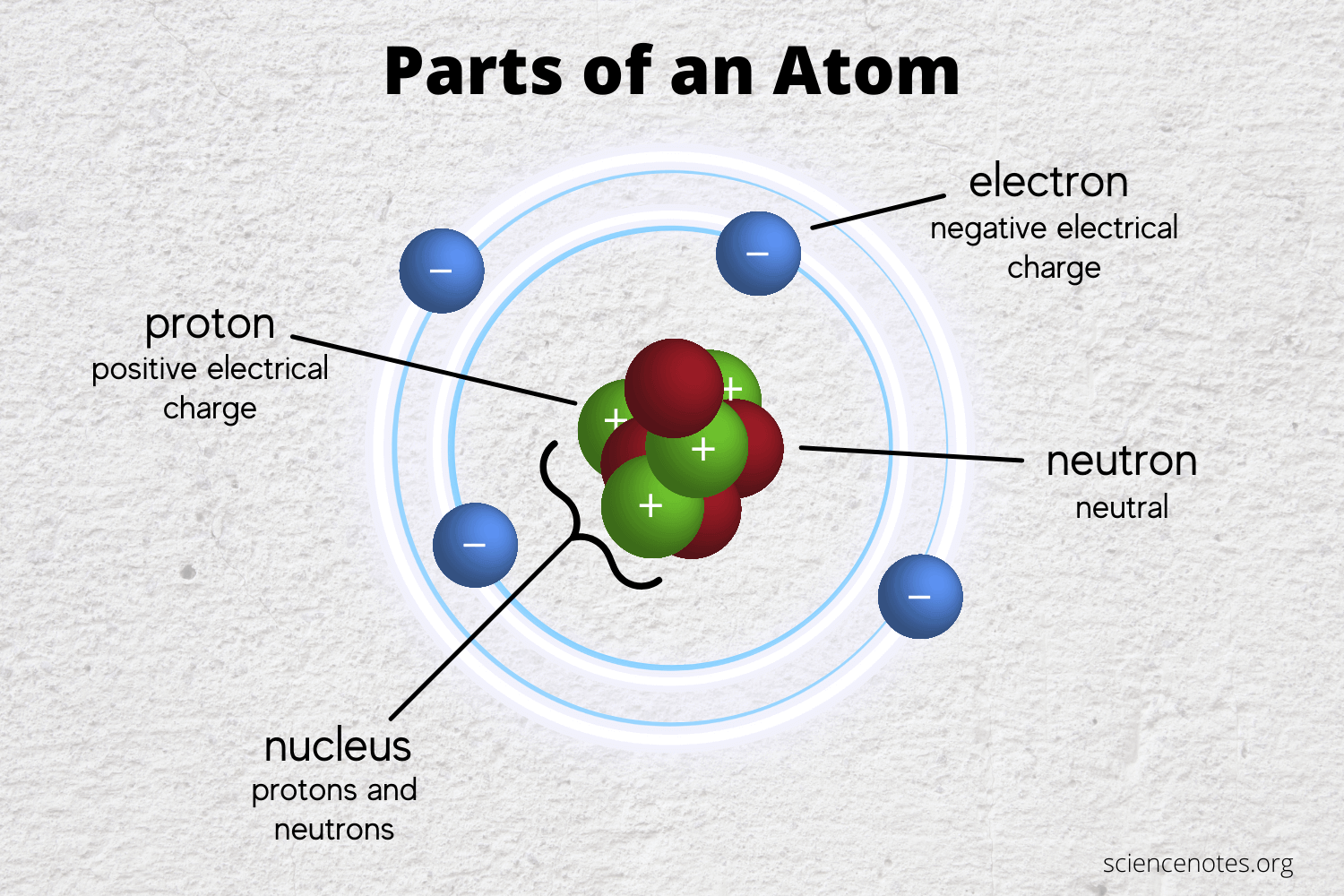
The number of protons determines what element the atom is. The nucleus contains Protons and Neutrons and makes up the vast majority of the atoms mass.
The Famous Types Of Subatomic Particles Praxilabs
Subatomic particles are particles that are smaller than the atom.

. Electrons are negatively charged having a charge of. A typical atom consists of three subatomic particles. List the 3 main types of subatomic particles and indicate the mass and electrical charge of each.
Protons neutrons and electrons are the three main subatomic particles found in an atom. 1 e textbf -1 e 1 e. 3Which subatomic particles contribute to atomic mass.
Protons have a positive charge. Electrons revolves around an atom. Protons are positively charged having a charge of.
The subatomic particles are protonsneutrons and electrons. An atom is a smallest particle of matter. These are three subatomic particles.
Form when two atoms have a very small nearly insignificant difference in electronegativity. 1Describe the subatomic particles that make up an atom. Opposite of the electron but equal in magnitude.
Electrons have negative - charge. Mats Persson Getty Images The atom is the smallest particle of matter than cannot be divided using a chemical means but atoms consist. Electrons have a.
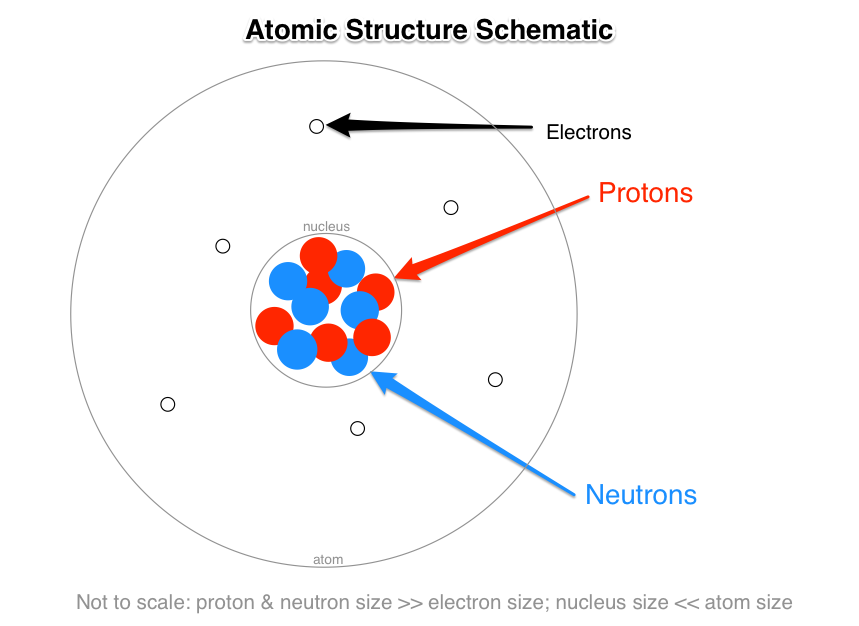
Protons and neutrons are nucleons. The electron cloud contains obviously Electrons. At the center of the atom is the.
Other particles exist as well such as alpha and beta particles which are discussed below. Terms in this set 67 Subatomic Particles. This is what allows elements to react.
3 main subatomic particles that form the atom. An atoms has two parts the nucleus and the electron cloud. Protons have a relative mass of 1 and a charge of 1 and they are found in the nucleus of an atom.
5Which subatomic particles contribute most directly in chemical bonding. 4 rows The main three subatomic particles are Protons electrons and neutrons. In contrast the electron has a negligible mass of 0005 amu.
Indicate the subatomic particle described by each of the statements. 4What is an Isotope. A statement may describe more than one particle.
An easy way to remember this is to remember that both proton and positive start with the letter P Neutrons have no electrical charge. The current model of the atom and the characteristics of each of the 3 subatomic particles are the electron cloud which contains 1 subatomic particle the electron which has negative charge and weights 12000 AMU the electron cloud surrounds the nucleus the nucleus contains two subatomic particles the proton which has positive charge and weights. Electron possesses a negative charge neutron has no charge electron has a mass slightly less than that of a neutron proton has a charge equal to but opposite in sign to that of an electron electron Answer Bank proton neutron profon.
List the 3 main types of subatomic particles and indicate the mass and electrical charge of each. Protons have a positive charge. Neutrons have no electrical charge.
Protons neutrons and electrons are the three main subatomic particles found in an atom. For simplicity we will use the amu unit for the three subatomics. It is present in the nucleus of.
3 Objective 6 Electron e -1 000055 0 Neutron n 0 100867 1 Proton p 1 100728 1 Mass Number Mass Daltons Name Symbol Charge 4 Objective 7 Describe the basic structure of the atom and be able to define the following terms. Protons neutrons and electrons. This is a positively charged particle that is present in the nucleus of atoms.
Within the nucleus protons and neutronsmake it. 3 Objective 1 Subatomic Particles Electron e -1 000055 0 Neutron n 0 100867 1 Proton p 1 100728 1 Mass Number Mass daltons Name Symbol Charge 4 Objective 2 Describe the basic structure of the atom and be able to define the. This particle has a charge of zero.
The three subatomic particles in an atom are protons neutrons and electrons. Particles that are smaller than the atom. Protons have a positive charge.
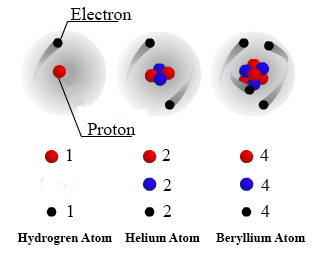
An atom consists of protons neutrons and electrons. Protons neutrons and electrons as seen in the helium atom below. Masses for the three subatomic particles can be expressed in amu atomic mass units or grams.
It has a charge of 16 1019C. The Bohr model shows the three basic subatomic particles in a simple manner. Protons neutrons and electrons are the three main subatomic particles found in an atom.
The three main subatomic particles of an atom are protons neutrons and electrons. In a atom subatomic partilcles are located in the nuclues andoutside of the nucleus. Subatomic particle Relative mass Relative charge.
Neutrons are neutral subatomic. Protons are positively charged species while neutrons are negatively charged species. Both neutrons and protons are assigned as having masses of 1 amu each.
2What subatomic particle is responsible for the Atomic number. Neutrons have a relative mass of 1 and no charge and they are also found in the nucleus of an atom. Include their charge and any contributions to atomic mass AMU.
Neutrons have no electrical charge. 1 e textbf 1 e 1 e.
What Are The Names Charges And Locations Of The Three Types Of Subatomic Particles That Make Up An Atom Socratic

Objectives 1 Name And Describe The Three Subatomic Particles In An Atom 2 Determine The Number Of Protons Neutrons And Electrons In An Atom Or Ppt Download
No comments for "What Are the 3 Subatomic Particles Describe Each"
Post a Comment